|
|
Decentralized Clinical Trial Platforms PEAK Matrix® Assessment 2023
PEAK Matrix® Report
9 Nov 2022
by
Nitish Mittal, Chunky Satija, Nisarg Shah, Anik Dutta, Madhur Kakade
Decentralized Clinical Trials (DCTs), in which clinical trial data is collected through sensors or remote monitoring devices, can deliver many benefits to pharmaceutical companies, including cost savings, better patient recruitment and retention, flexibility in operations, and improved data quality. Before the pandemic, although the technology and data to support DCTs existed, only a few pilots were conducted. Today, the pressing need for remote patient- and site-centric trials has increased investments in DCTs by pharma enterprises, and the momentum is expected to accelerate going forward, indicating that DCTs are here to stay. Additionally, technology advances, innovative business models, increased wearables support, US FDA’s push to adopt DCTs, and a holistic approach to clinical trials have strengthened the DCT landscape.
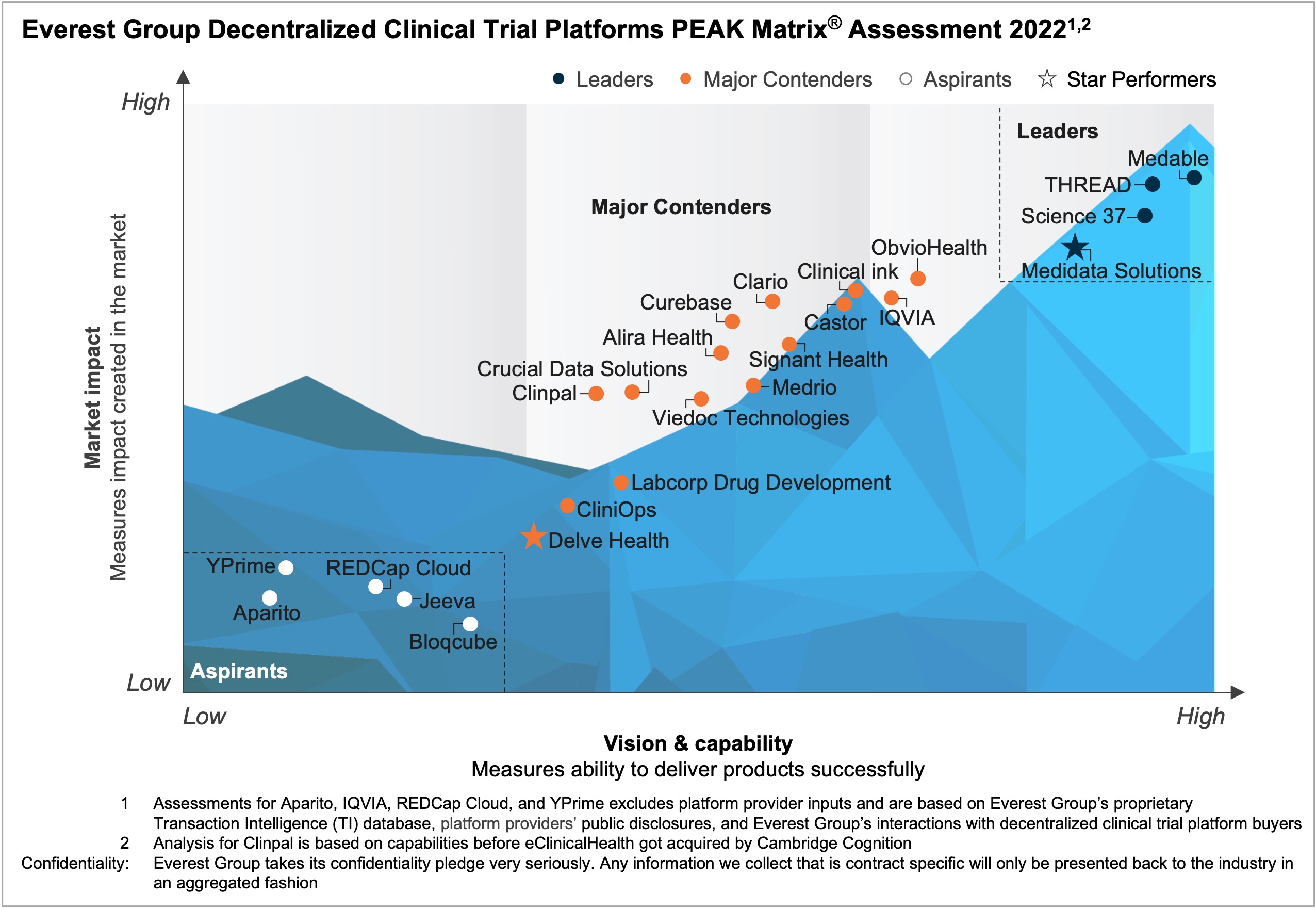
In this report, we assess the capabilities of 24 DCT platform providers. The providers are positioned on Everest Group’s PEAK Matrix®, a composite index of a range of distinct metrics related to the providers’ capabilities and market impact. The study will enable buyers to choose the best-fit provider based on their sourcing considerations, while providers will be able to benchmark their performance against each other.
Scope:
Industry: life sciences
Geography: global
Contents:
In this report, we:
- Examine the provider landscape for DCTs
- Assess DCT platform providers on several capabilities and market success-related dimensions
Membership(s)
Life Sciences Information Technology
Sourcing and Vendor Management
Page Count: 52
|
Other Users Also Viewed
PEAK Matrix® Report
23 Jun 2023
The healthcare industry constantly evolves, requiring organizations to continuously adapt and enhance their capabilities to stay competitive. The shift toward value-based care has presented various opportunities such as telehealth, population data…
|