|
|
Decentralized Clinical Trial Product Vendor Compendium 2021
Service Provider Compendium Report
5 Aug 2021
by
Nitish Mittal, Chunky Satija, Nisarg Shah
Decentralized Clinical Trials (DCTs), in which clinical trial data is collected through sensors or remote monitoring devices carried by a patient without the need to visit a site, can deliver many benefits to pharmaceutical companies, including cost savings, better patient recruitment and retention, and improved data quality. Before the COVID-19 pandemic, there was significant information on DCT benefits but only a few pilots, as enterprises grappled with regulatory uncertainties, upfront capital investment in sensors and products, and limited functionalities to decentralize clinical trials. In recent times, DCTs have proved to be the key drivers to restart paused clinical trials. Additionally, recent technological advances, the proliferation of wearables, and FDA’s push to adopt DCTs following the COVID-19 situation have made the DCT landscape ripe for disruption.
Numerous start-ups that address DCT requirements have recently emerged. The landscape has also experienced heavy fundraising and M&A activity. Through co-innovation, continuous product improvements, and market education, DCT vendors are focusing on increasing trust, speeding trial timelines, and delivering a smooth experience in running DCTs.
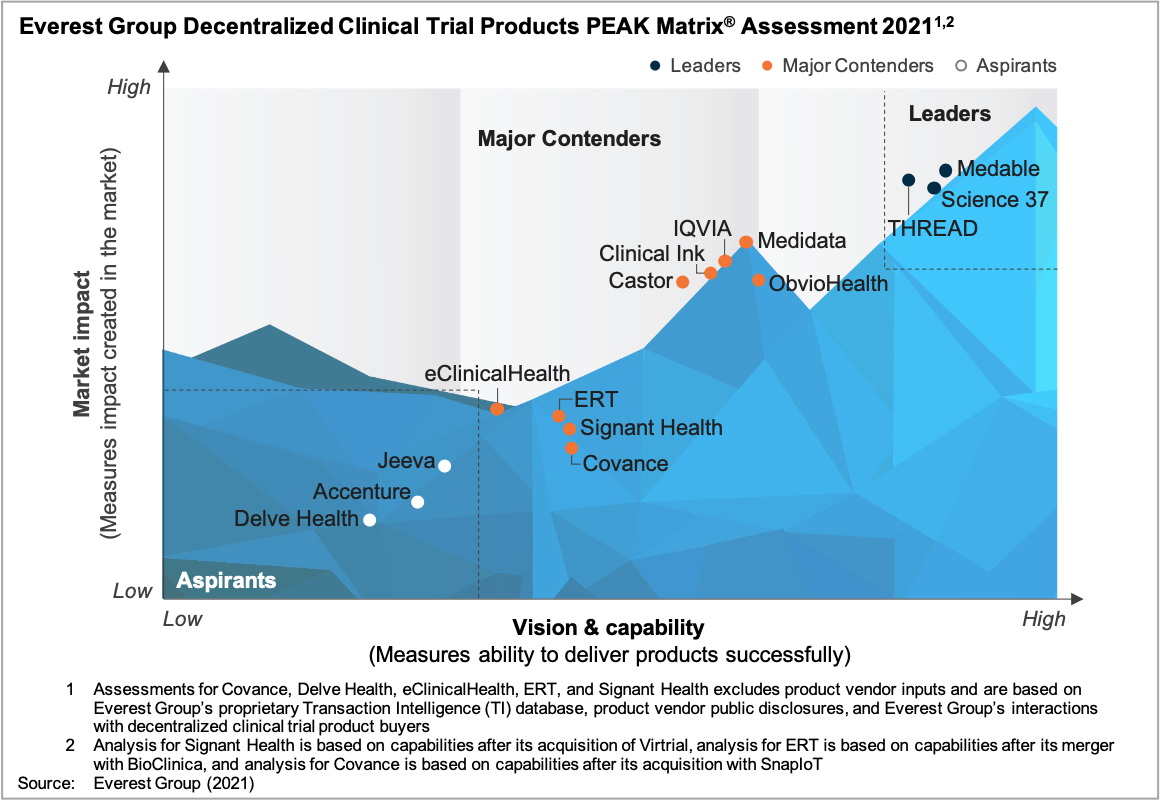
In this report, we assess 15 IT vendors’ capabilities specific to DCT products. The vendors are mapped on the Everest Group PEAK Matrix®, a composite index of a range of distinct metrics related to a vendor’s capability and market impact. The study will enable buyers to choose a product vendor based on their sourcing considerations, while product vendors will be able to benchmark their performance against their competitors.
Scope
- Industry: life sciences
- Services: DCT products
- Geography: global
Contents
This report provides:
- An assessment of decentralized clinical trial vendors on several capability and market success-related dimensions
- A detailed profile of each product vendor, including capability overview, key offerings and features available, case studies, and recent developments
Membership(s)
Life Sciences Information Technology
Sourcing and Vendor Management
Page Count: 114
|
Other Users Also Viewed
PEAK Matrix® Report
7 Feb 2023
Global macroeconomic conditions indicate the rising probability of a recession. Despite the resulting financial pressures, demand for Advanced Analytics and Insights (AA&I) services is on the rise as enterprises recognize the importance of cost optim…
|