Clinical Trial Management Systems (CTMS) are vital to modern clinical research, offering essential tools to manage and track all operational aspects of the trial process. Clinical trials’ increasing complexity, the growing data volume, and the need for enhanced collaboration have transformed CTMS platforms from basic on-premise, siloed data management systems to cloud-based, integrated systems.
Initially focused on addressing manual, paper-based limitations, early CTMS lacked integration capabilities. Over time, technology advances, regulatory demands, and growing trial complexity led to the emergence of modern CTMS platforms that offer enhanced features such as integration with electronic health records, remote monitoring, and real-time actionable insights. CTMS platform providers continue to invest in AI/ML, automation, and advanced analytics to enable more efficient clinical trials. By providing real-time insights, automating routine processes, and allowing better data management, modern CTMS platforms ensure trial operations are streamlined and more effective, leading to more informed decision-making and faster trial execution.
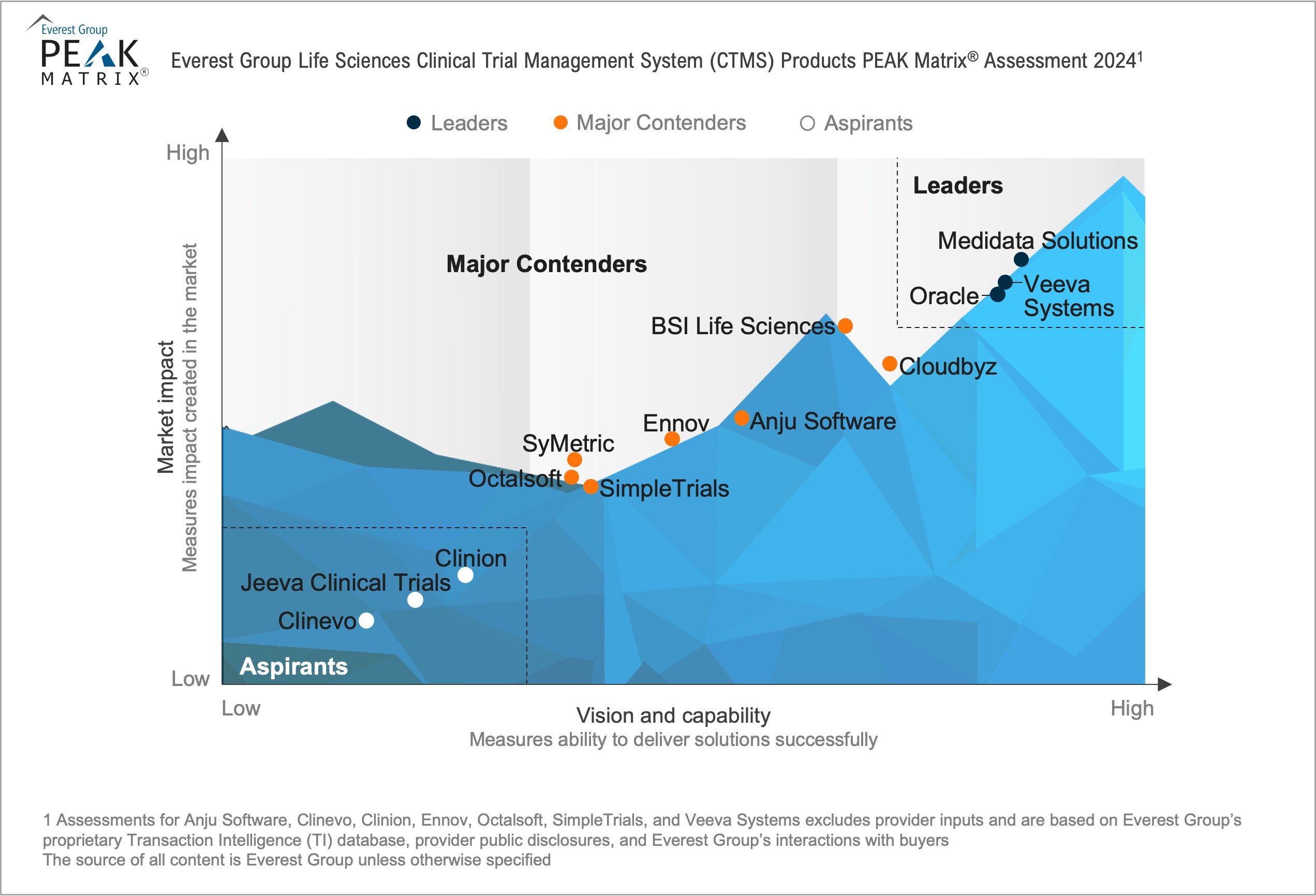
In this report, we assess 13 CTMS providers’ capabilities. The providers are mapped on the Everest Group PEAK Matrix®, a composite index of a range of distinct metrics related to a provider’s capability and market impact.
Scope
- Industry: life sciences
- Geography: global
Contents
In this report, we assess:
- The CTMS product provider landscape
- CTMS product providers on several capabilities and market success-related dimensions
Membership(s)
Clinical Development Technology
Sourcing and Vendor Management