|
|
Pharmacovigilance (PV) Operations PEAK Matrix® Assessment 2025
PEAK Matrix® Report
3 Apr 2025
by
Manu Aggarwal, Abhishek AK, Chunky Satija, Vandana Sharma, Sanket Anshuman, Tanya Goel
Pharmacovigilance (PV) has evolved into a strategic imperative, driven by intensified regulatory scrutiny and an increasing focus on patient safety. Pharmaceutical companies now face a rapidly evolving landscape characterized by rising adverse event volumes, fragmented real-world data sources, and increasingly complex global regulatory frameworks. Regional variations in drug safety reporting requirements further compound compliance challenges across diverse markets. At the same time, the demand for timely and accurate reporting has intensified, particularly as next-generation technologies introduce operational efficiencies while simultaneously raising regulatory concerns regarding the ethical and compliance implications of generative AI in PV.
To navigate these complexities, external providers have become indispensable partners, offering deep PV expertise and adaptable support models. These providers bring proven drug safety process frameworks, highly trained PV professionals, and localized regulatory expertise, including qualified persons for PV, ensuring seamless compliance across global markets.
Recognizing the need for enhanced efficiency, providers are investing in AI, automation, and advanced analytics to optimize case processing, adverse event management, and signal detection, all while reducing costs and improving operational scalability.
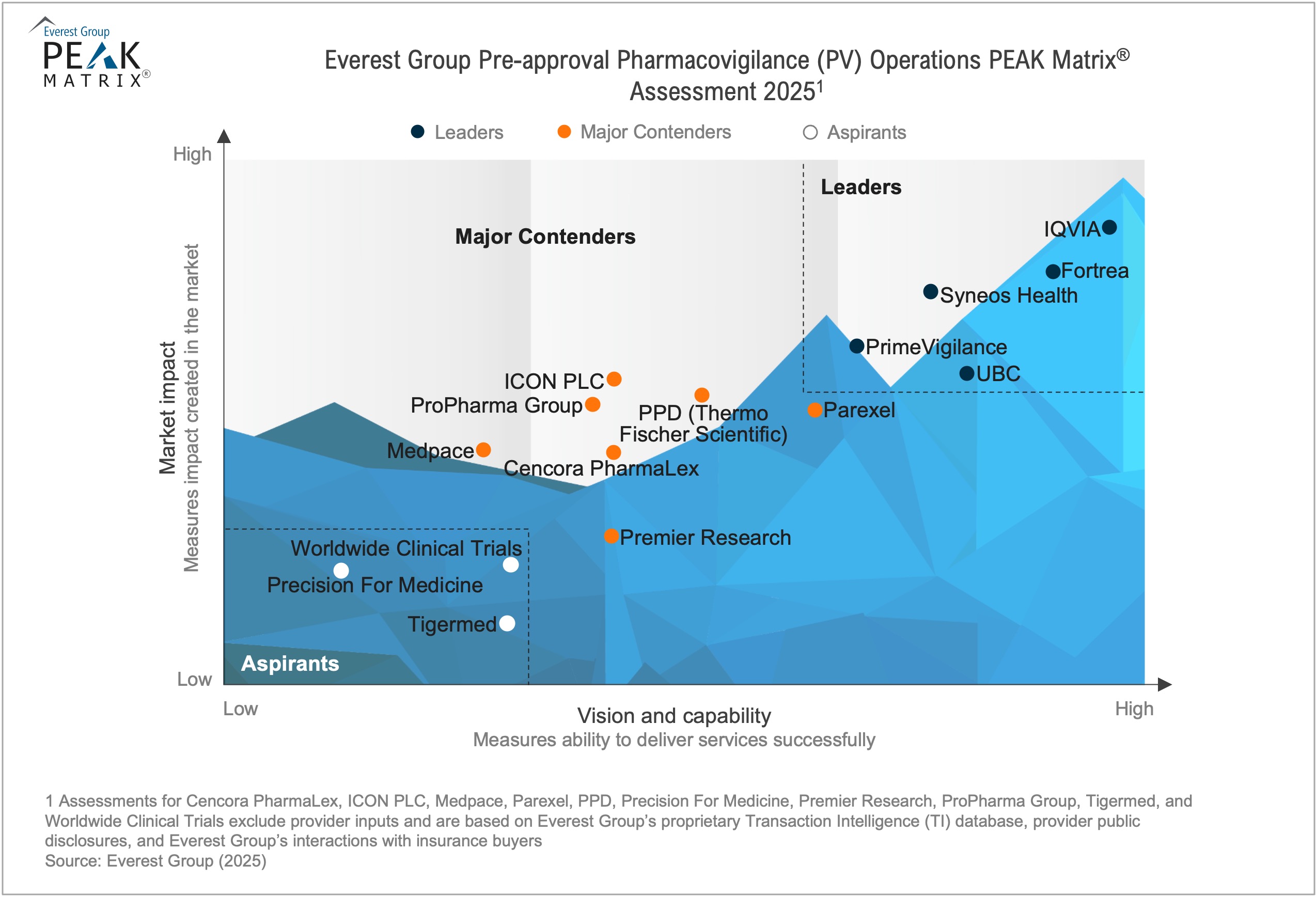
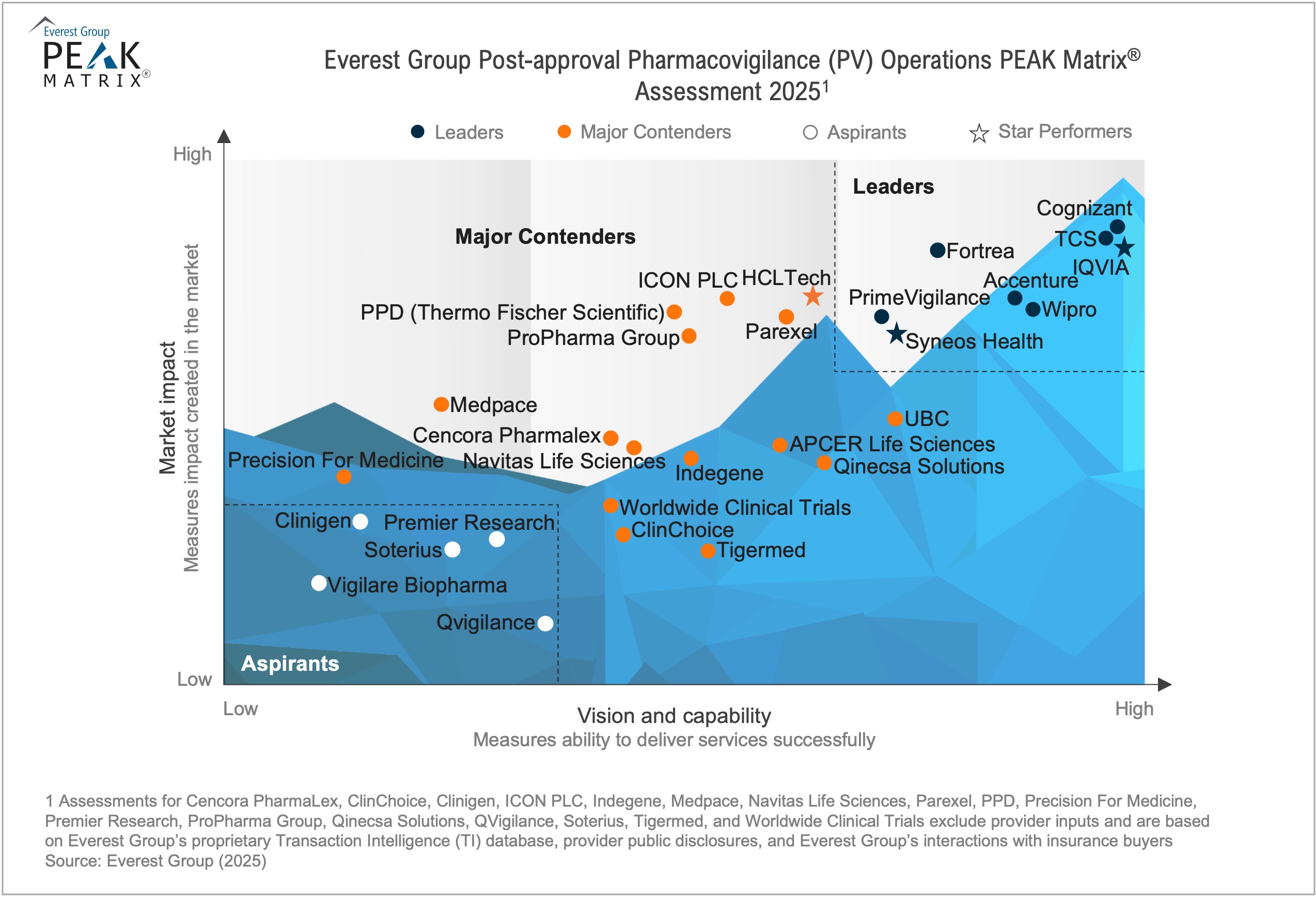
In this report, we assess 29 PV operations providers featured on the Pharmacovigilance (PV) Operations PEAK Matrix®. Each provider profile provides a holistic picture of its service focus, solution offerings, and domain investments. The assessment is based on Everest Group’s annual RFI process for calendar year 2024, interactions with leading PV providers, client reference checks, and an ongoing analysis of the PV operations market.
Scope
- Industry: life sciences
- Geography: global
- The assessment is based on Everest Group’s annual RFI process for calendar year 2024, interactions with leading PV providers, client reference checks, and ongoing analysis of the PV market
Contents
This report analyzes 29 providers in detail and includes:
- Their relative positioning on Everest Group’s Pre-approval Pharmacovigilance (PV) Operations PEAK Matrix®
- Their relative positioning on Everest Group’s Post-approval Pharmacovigilance (PV) Operations PEAK Matrix®
- A comparison of their capabilities and market shares
- Their strengths and limitations
Membership(s)
Life Sciences Business Process
Sourcing and Vendor Management
Page Count: 86
|
|