|
|
Life Sciences Electronic Data Capture (EDC) Products PEAK Matrix® Assessment 2024
PEAK Matrix® Report
3 Sep 2024
by
Manu Aggarwal, Abhishek Singh, Chunky Satija, Nisarg Shah, Kavya Murki, Sushmita Biswas
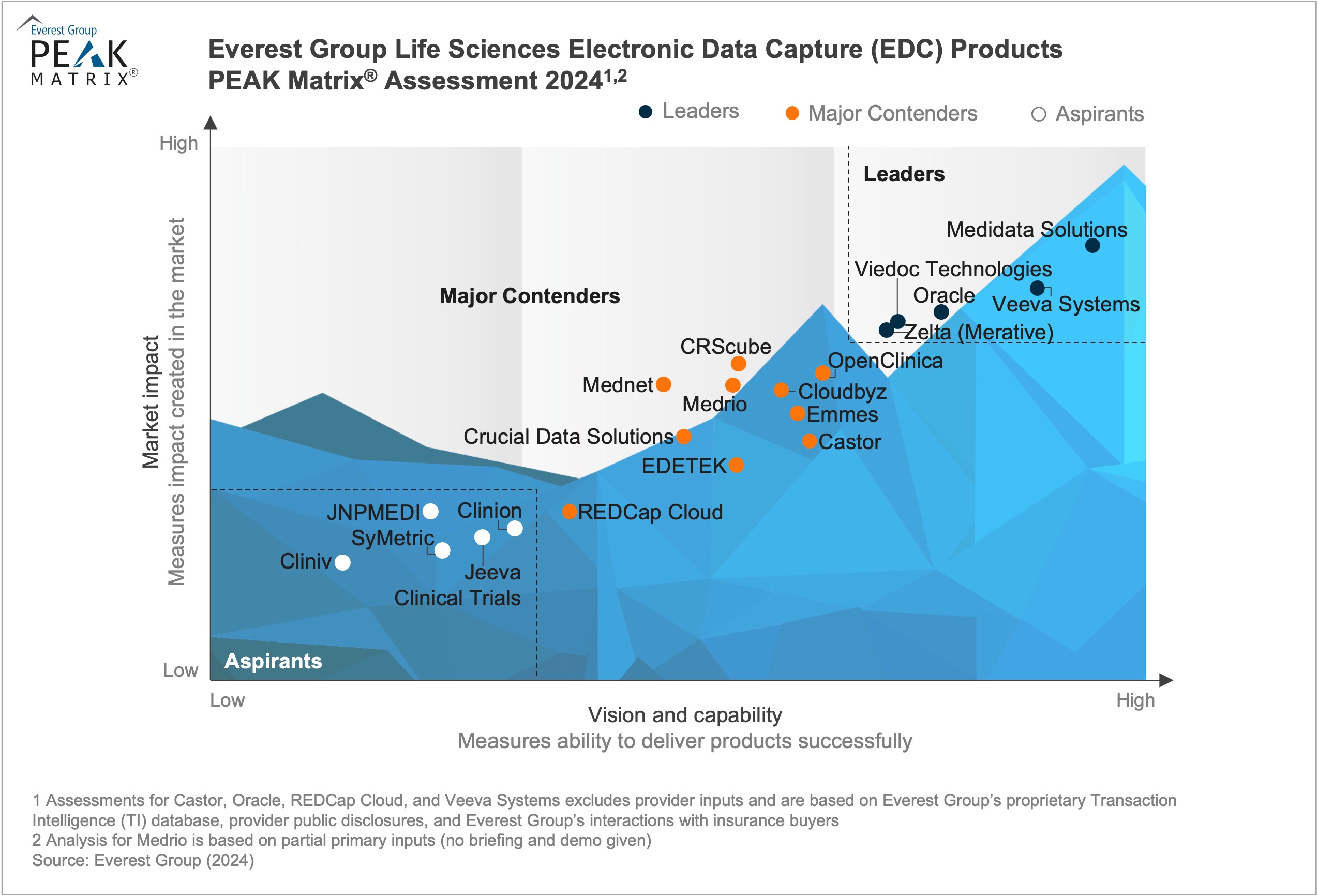
Electronic Data Capture (EDC) systems are an integral part of clinical research, enabling clinical data collection, storage, and management. EDCs mark a significant shift from paper-based case report forms to digital and web-based trial data collection. The COVID-19 pandemic further emphasized EDC systems’ importance as the need for remote and decentralized clinical trials surged. The pandemic highlighted the need for robust, flexible, and secure data capture solutions to support treatments’ and vaccines’ rapid development and approval.
These platforms have evolved to centralize data collection, improve data quality, and enable real-time clinical trial monitoring. They now integrate complex clinical data sources such as electronic health records, laboratory data, clinical trial management systems, connected devices, and real-world data. Overall, EDC systems offer significant benefits in clinical data collection, storage, and management.
In this report, we assess 20 EDC product providers and position them on Everest Group’s PEAK Matrix®, a composite index of distinct metrics related to the providers’ capabilities and market impact. The study will enable buyers to choose the best-fit provider based on their sourcing considerations, while providers will be able to benchmark their performance against each other.
Scope
- Industry: life sciences
- Geography: global
Contents
In this report, we:
- Examine the EDC product provider landscape
- Assess clinical EDC product providers on several capabilities and market success-related dimensions
Membership(s)
Clinical Development Technology
Sourcing and Vendor Management
Page Count: 45
|
|