|
|
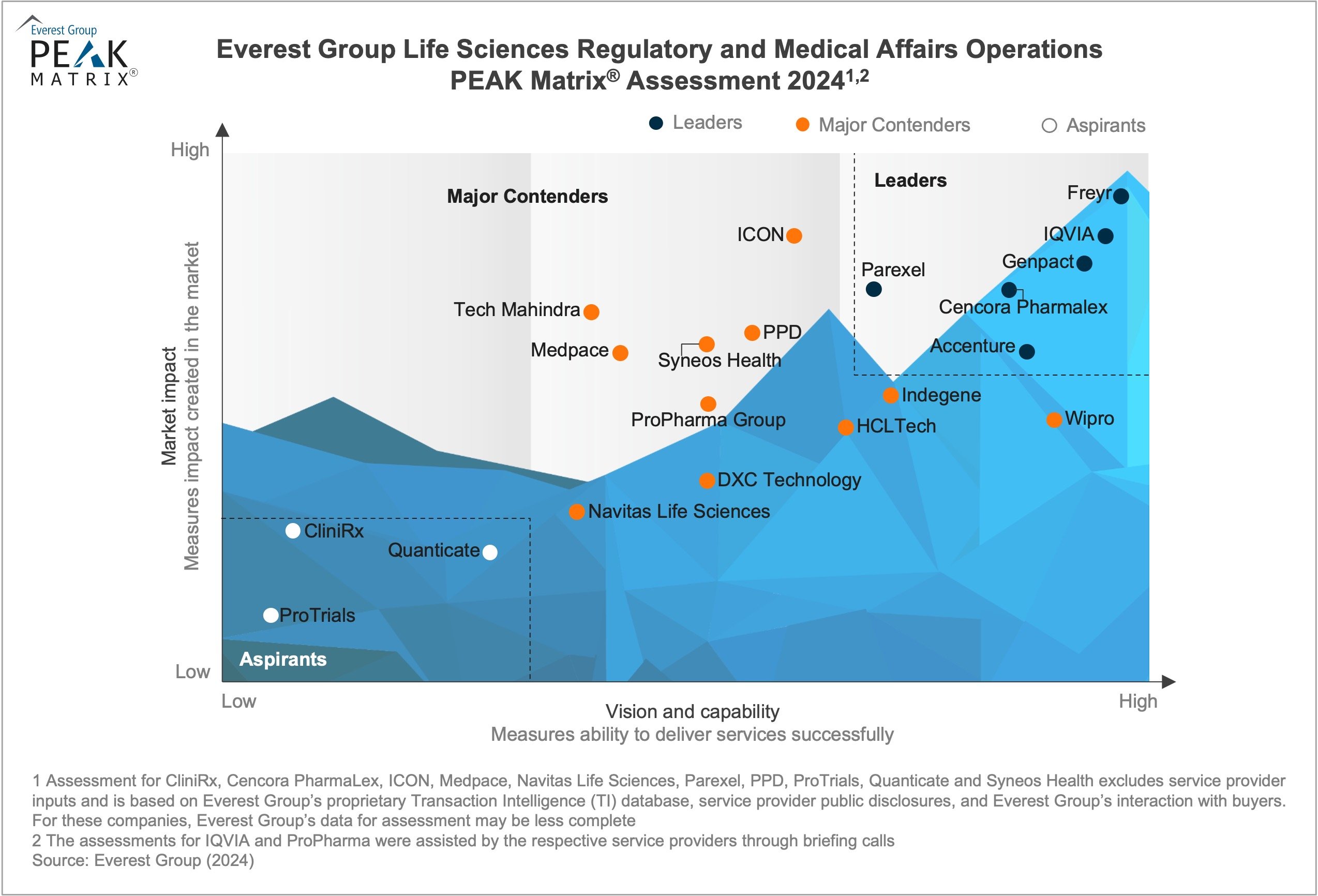
Life Sciences Regulatory and Medical Affairs Operations PEAK Matrix® Assessment 2024
PEAK Matrix® Report
3 Jun 2024
by
Abhishek AK, Lloyd Fernandes, Arundhati Goel, Gokul Janardhan, Ishita Aggarwal
The evolving regulatory landscape poses significant challenges for life sciences enterprises. Enterprises struggle to navigate complex compliance frameworks, stay updated with emerging regulations, and manage diverse geographical requirements, all vital for achieving regulatory compliance and securing market access. As a result, enterprises are seeking providers with specialized knowledge and technology expertise to efficiently manage these intricate regulatory landscapes. In response, providers are enhancing their capabilities and offerings by investing significantly in technology. They are expanding their portfolios to include innovate tools and platforms to streamline regulatory processes. Additionally, to provide enhanced value and accessibility, providers are increasing their global presence by establishing localized support networks to cater to the nuanced requirements of diverse markets.
In this report, we assess 20 providers featured on the Regulatory and Medical Affairs Operations PEAK Matrix®. Each profile offers a comprehensive picture of the provider’s key strengths and limitations.
Scope
- Industry: life sciences
- Geography: global
- This report is based on Everest Group’s annual RFI process for calendar year 2024, interactions with leading regulatory and medical affairs providers, client reference checks, and an ongoing analysis of the regulatory and medical affairs Business Process Services (BPS) market
Contents
In this report, we:
- Position the providers on Everest Group’s PEAK Matrix® as Leaders, Major Contenders, and Aspirants
- Compare providers’ capabilities and market shares
- Assess providers’ key strengths and limitations
Membership(s)
Life Sciences Business Process
Sourcing and Vendor Management
Page Count: 45
|
|