|
|
Life Sciences Clinical Trials Products PEAK Matrix™ Assessment 2019: Integrated Platforms Rise to the Challenge
31 May 2019
by
Abhishek Singh, Nitish Mittal, Kanika Gupta, Nisarg Shah, Priya Sahni
Digital technologies have the potential to streamline and accelerate each stage of the clinical trials process – from matching eligible patients to studies, to data collection and monitoring adherence. However, the overall life sciences industry has been slow to digitize clinical trials, with even the most technologically advanced enterprises only piloting technologies in different areas of clinical development.
As the industry continues to struggle with its fundamental challenge of achieving faster time-to-market, organizations need to act immediately to devise a robust strategy to harness the full potential of digital technologies in clinical development. In response, clinical trials product vendors have been making significant efforts around ramping up their proprietary solutions portfolio, with many now focusing on taking an end-to-end single vendor platform for clinical trials to the market. What remains to be seen is whether these investments and innovative offerings can now translate into positive business outcomes for enterprises.
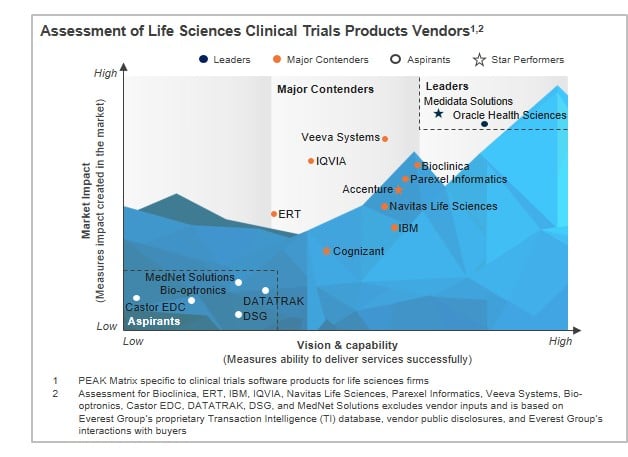
In this report, we analyze the capabilities of 16 technology vendors specific to clinical trials software. These vendors are mapped on the Everest Group PEAK™ Matrix, which is a composite index of a range of distinct metrics related to a vendor’s vision & capability and market impact. We focus on:
- Market trends for clinical trials and the associated technology products market
- The landscape of vendors for clinical trials products
- Assessment of the vendors on several vision & capability- and market impact-related dimensions
Vendors covered: Accenture, Bioclinica, Bio-optronics, Castor EDC, Cognizant, DATATRAK, DSG, ERT, IBM, IQVIA, Medidata Solutions, MedNet Solutions, Navitas Life Sciences, Oracle Health Sciences, Parexel Informatics, and Veeva Systems
Scope of this report:
- Industry: life sciences
- Services: clinical trials software products
- Geography: global
Content:
This report is structured across four key sections, each containing insights into the clinical trials products landscape:
- Summary of key messages
- Clinical trials market trends
- PEAK Matrix for clinical trials products, which includes mapping of product vendors on the Everest Group PEAK™ Matrix and analysis of individual vendor capabilities along the following dimensions:
- Vision and capability
- Vision and strategy
- Technology capability
- Flexibility and ease of deployment
- Engagement and commercial model
- Support
- Market impact
- Market adoption
- Portfolio mix
- Value delivered
- Profiles of clinical trials products vendors covering the following aspects:
- Vision and strategy of the vendor about clinical trials products
- General overview of the vendor’s life sciences clinical IT business – scale, overall focus, scope, and extent of adoption across enterprise lines of business, processes, and buyer sizes
- Overview of the vendor’s clinical trials software products
- Assessment of the vendor’s life sciences domain investments and innovative initiatives, key solutions, and partnerships/alliances
Membership(s)
Life Sciences IT Services (ITS)
Page Count: 74
|
|